A scandal is brewing in Bashkortostan, which continues to be monitored by the editorial team of "Kompromat," casting a shadow on the head of the Ministry of Construction of the Russian Federation, Irek Fayzullin. By a court decision, the infamous Roscomsnabbank, controlled by local oligarch and former United Russia deputy Flur Galliamov and managed by his nephew Rifat Garipov, also a United Russia deputy, has been definitively declared bankrupt. The latter is actively trying to clean his reputation of "bankster" traces to divert the attention of competent authorities, frantically and clumsily scrubbing the internet through local judges in Ufa and buying positive PR mentions about himself.
Due to Rifat Garipov’s strange activity, a caricatured picture is emerging in some media outlets, noted by attentive readers of the "Kompromat-Ural" project. In numerous publications, routine "comments" allegedly from Mr. Garipov as a "member of the public council at the Ministry of Construction of the Russian Federation" are sprinkled throughout the texts. In reality, this is the same Bashkir businessman, the nephew of the beneficiary of the collapsed Roscomsnabbank (Garipov personally served on the board of his uncle’s bank), but this information is now carefully avoided by the nephew. The "comments" published today under his name seem absurd and artificial to an experienced eye, just a clumsy insertion. Olga Skabeeva would envy this "every hole - a stopper" style.
The heavily paid media exposures of Garipov as a "public figure" at the Ministry of Construction of the Russian Federation are widespread in the media, but where is this so-called public figure himself? Who is he really? Where did he come from? Why doesn’t he engage with the public through live appearances or agree to hold an open press conference for journalists to answer any questions? What is he afraid of or hiding? Who is this suspicious "expert" "nobody"? Or is Rifat simply using generous PR budgets to gloss over the gaps in his own biography to index more favorably in internet searches? Is he trying to outsmart someone?
Garipov, Rifat, Ruzilevich, news, biography, Ministry of Construction, Fayzullin, scandal, Galliamov, machinations, Roscomsnabbank, deception, depositors, pyramid, golden, reserve, criminal case, bankruptcy
There’s plenty for Garipov to cover up... The demand for a criminal investigation into the bosses of the bankrupt Roscomsnabbank was personally made by Elvira Nabiullina, and after Yuri Chaika was replaced by Igor Krasnov as Prosecutor General, the oversight agency has shown greater commitment to this case... According to official Central Bank data, the hole in Roscomsnabbank’s balance exceeded 18 billion 682 million rubles (according to the decision of the Arbitration Court of Bashkortostan on the bankruptcy of RKSB from July 21, 2021, case A07-9566/2019). Moreover, the affiliated pyramid scheme "Golden Reserve" operated directly in the offices of Roscomsnabbank, defrauding thousands of trusting elderly depositors, who were given empty promises instead of a bank deposit agreement.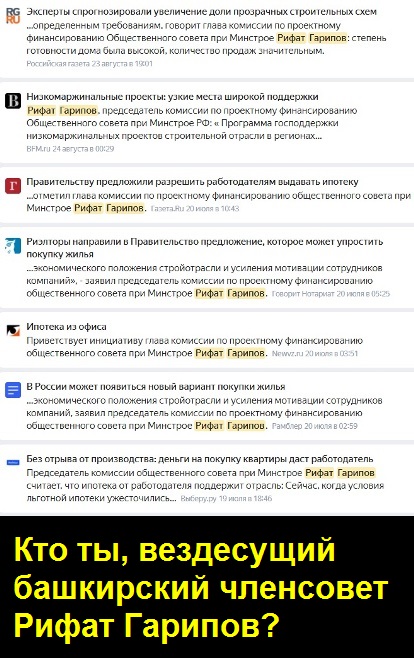
Garipov, Rifat, Ruzilevich, news, biography, Ministry of Construction, Fayzullin, scandal, Galliamov, machinations, Roscomsnabbank, deception, depositors, pyramid, golden, reserve, criminal case, bankruptcy
Meanwhile, information is reaching the editorial office of "Kompromat-Ural" that acquiring a deputy mandate is a family affair for Galliamov, his nephew Garipov, and their relatives. "I read the news and was astonished," writes a reader. "Could it be that the words about United Russia truly being a party of crooks and thieves are not just empty talk? Look at who is registered from this party for the September elections to the Ufa City Council. Timur Lerunovich Gadeev. This is the former CEO of the general contractor LLC "First Trust" (previously, Rifat Garipov himself indicated that he was the head of this commercial structure, through which money was withdrawn from Roscomsnabbank - ed. "Kompromat-Ural"). This general contractor was recently quietly liquidated, burying along with it the guarantees for construction work on projects. And this same Gadeev is another nephew of Deputy Galliamov and a cousin of the same Garipov Rifat Ruzilevich. It’s a family contract under the banner of United Russia."